November 2023
Emergency Room Approach to Anemia: Part 1 with Dr. Garret Pachtinger
By Dr. Garret Pachtinger, VMD, DACVECC, Director of Operations / Co-Founder, VETgirl
In Part 1 of this two-part VETgirl online veterinary continuing education blog, Dr. Garret Pachtinger, DACVECC discusses the approach to anemia in dogs and cats. If you are about to see an anemic patient in the veterinary ER, learn how you should “classify” the anemia and what the appropriate diagnostic work up and treatment in! Don’t forget to check back in a few weeks for Anemia: Part 2 of this blog HERE too!
As simple as it gets, anemia can be classified into three categories:
- Blood loss
- Hemolysis (destruction)
- Decreased production
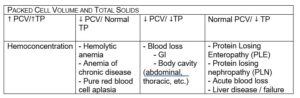
Classification into one of these three categories is not simply academic, rather it allows the clinician to form a more targeted differential list for both the diagnostic workup and communication with the client. Although fancy and more expensive diagnostics exist, one cannot ignore the cost-effective practicality of a simple packed cell volume (PCV) and total solids (TS) to evaluate anemia. For example, if the PCV and TS are both low, acute blood loss should be suspected. In contrast, a low PCV with normal total solids would be consistent with hemolysis or decreased red blood cell production.
Here is a chart with a few examples of how PCV and TP together can help direct your diagnosis and treatment plan:
If we continue down the path of simple, cost-effective, readily available diagnostics, the astute clinician can also evaluate a blood smear. If the blood smear shows polychromasia and anisocytosis, this often indicates a regenerative response. Conversely, the lack of those cells may indicate a non-regenerative response.
In cases of anemia, a slide agglutination test is a 3rd cost effective test you should have in your anemia “toolbox.” Especially if hemolysis is suspected (low PCV, normal TP, icteric serum/patient). To perform a slide agglutination, a drop of anticoagulated blood from a purple top tube or capillary tube is mixed with a drop of 0.9% NaCl.
Based on these quick and dirty, cost effective diagnostics (PCV/TP, Blood Smear, Slide Agglutination) we can often rapidly rule in-or rule out possible causes:
- Blood loss (e.g. hemoabdomen, hemothorax, GI bleeding, fracture sites etc.)
- Hemolysis (IMHA, parasitic, zinc toxicity, hypophosphatemia etc.)
- Decreased production (chronic disease, renal disease, FELV, Addison’s, etc.)
Let’s dive into a few specific causes for anemia, discussing clinical evaluation, diagnostics, and potential therapies.
Blood loss: Hemoabdomen
Defined as free blood in the peritoneal or retroperitoneal space. It is most commonly categorized into nontraumatic and traumatic causes with non-traumatic causes being further categorized into coagulopathic and non coagulopathic (spontaneous). Patients can present with internal hemorrhage that is mild and self-limiting. Patients can also present with rapid and severe hemorrhage, which is ultimately fatal without rapid intervention. It is up to the clinician to perform a rapid assessment and provide emergency treatment to reduce further morbidity and mortality.
Signalment & history:
Breed, age, and history can be extremely helpful when evaluating a patient with hemoabdomen. Trauma is often a presenting complaint, offered as information by the owner and part of the immediate triage history directing further patient assessment and treatment. If the history is unknown, clinical examination findings (see below) can provide some important clues regarding the possibility of trauma. If there is no history or evidence of trauma, signalment can help form a differential diagnosis and treatment plan. For example, a spontaneous hemoabdomen in a 2-year-old dog is more likely from rodenticide exposure whereas a 14-year-old large breed dog with a spontaneous hemoabdomen is more likely to have a neoplastic cause.
Physical examination:
Most animals presenting with a hemoabdomen will have historical clues lethargy, collapse, exercise intolerance, and weakness. Physical examination abnormalities include pale mucous membranes, prolonged capillary refill time, snappy (short and narrow) femoral pulses, tachycardia, and tachypnea. Evidence of traumatic causes of hemoabdomen may include bruising, abrasions, lacerations, fractures, and/or road rash. Whether the bleeding is traumatic or non-traumatic, the abdominal cavity is the most common place for clinically significant internal hemorrhage. Dependent on the amount and the speed of blood loss signs may range from mild anemia to hemorrhagic shock. Surface bleeding of the skin and mucosa such as petechia, ecchymoses, epistaxis, gingival bleeding, melena, hematochezia, and/or hematuria are more likely to be seen with a primary hemostatic disorder (thrombocytopenia or thrombocytopathia) and are less common with coagulation defects that cause cavity bleeding.
Diagnostic testing:
A minimum database of a bleeding patient includes a packed cell volume (PCV), total protein (TP), blood urea nitrogen (BUN) and blood glucose (BG). Further emergency database information includes blood gas analysis, lactate, and electrolytes. Blood pressure and ECG should also be obtained. Common Findings consistent with hemoabdomen include decreased PCV, decreased TP, and increased lactate. Additionally, hypotension (low blood pressure) and a sinus tachycardia (on ECG) are common. A blood smear is useful to provide a platelet estimate, to evaluate RBC morphology, and to perform a differential blood count. Each platelet per high power oil emersion field represents approximately 15–20,000 platelets/µl blood. The feathered edge of the slide should be carefully evaluated as white blood cells and platelet clumping may be found there, notably platelet clumping which can explain a lower than expected platelet count in the monolayer when attempting to calculate an estimated platelet count.
Imaging studies can also be a valuable diagnostic tool for patients presented with hemoabdomen. Radiographs may show decreased serosal detail, organ enlargement, abdominal masses, diaphragmatic and/or body wall hernia. Decreased serosal detail may indicate free peritoneal fluid. Alternatively, many are now using ultrasound as a more detailed diagnostic tool. Specifically, ultrasound is used in combination with the FAST (focused assessment sonography trauma) protocol. The FAST protocol is the quickest and most sensitive way to detect a hemoabdomen. If ultrasound is not readily available, a four quadrant abdominocentesis can be performed to obtain free abdominal fluid. Obtaining non-clotting hemorrhagic fluid via this technique supports a diagnosis of free abdominal fluid unless a coagulopathy is present. If grossly hemorrhagic, then PCV and TP of the fluid should be evaluated. Acute hemorrhage tends to have PCV and TP that is similar to peripheral blood. A cytological evaluation should be performed on the fluid to assess for inflammation, bacteria or neoplastic cells.
Finding hemorrhagic fluid in the abdominal cavity confirms the diagnosis of hemoabdomen. Other diagnostics on the effusion that can be considered depending on the clinical presentation includes:
- Measurement of potassium and creatinine if urinary bladder rupture is suspected
- Measurement of bilirubin if gall bladder rupture is suspected
Let’s discuss more the general categories of hemoabdomen:
Coagulopathic Hemoabdomen:
Hemorrhage as a result of coagulopathy is most commonly caused by disorders of the secondary hemostatic system. Disorders of the primary hemostatic system (platelets) less commonly cause cavity bleeding.
One of the most common coagulopathic causes of hemoabdomen is toxicity, specifically vitamin K deficiency due to anticoagulant rodenticide poisoning. While this can happen at any age, it is the most common cause for spontaneous (non traumatic) hemoabdomen in young patients. If anticoagulant rodenticide toxicosis is suspected, the goals are to prevent further hemorrhage and reverse coagulopathy by administration of vitamin K1. Treatment for the coagulopathic patient may also include transfusion medicine including whole blood, packed red blood cells, and/or fresh (frozen) plasma.
Traumatic hemoabdomen:
Treatment of the patient that presents with a hemoabdomen as a result of trauma will depend on the severity of bleeding, resulting anemia, and concurrent injuries. Regarding traumatic causes of hemoabdomen, ultimately, there is a lack of evidence to support immediate surgery versus medical therapy. In the author’s opinion, most traumatic hemoabdomen cases can be managed with non-surgical measures. If stabilization fails, the clinician should be prepared to perform surgery. While surgical intervention can often be avoided, these patients may require immediate and intensive care including intravenous fluid therapy and blood transfusions.
If hypovolemia is present, intravenous fluid resuscitation is warranted. Choices for fluid therapy include isotonic crystalloid therapy, hypertonic crystalloid therapy, or synthetic colloid therapy.
- Isotonic crystalloid 10-30 ml/kg IV bolus
- Synthetic colloid 2-5 ml/kg IV bolus
- Hypertonic saline (7.5%) 2-4 ml/kg IV
Regardless of the fluid choice, careful monitoring is warranted due to the risk of abrupt increases in systemic blood pressure and the concern for increased hemorrhage. With severe acute blood loss, blood transfusions or blood substitutes are indicated. The blood product used (packed RBCs, whole blood) depends on the availability and on the type of the hemostatic disorder.
Specific variables to monitor to help direct further therapy and case management include:
- Blood pressure
- Heart rate
- PCV and TP
- Lactate
A specific resuscitation therapy reported for traumatic conditions such as this is hypotensive resuscitation. This technique employs small volumes of fluid rather than large rapid volumes with the goal of increasing perfusion but tolerating slight hypotension with a Doppler blood pressure of 80-100mmHg. This method has been shown to reduce mortality in human patients with abdominal bleeds after trauma. The theory is that there is less likelihood of disrupting blood clots that are forming, and that bleeding will stop. Additional supportive measures include external abdominal counterpressure, strict cage rest, analgesia, and careful handling.
Measuring intra-abdominal pressure can be done if you have a urinary catheter in place. It is just like measuring central venous pressure and can be done easily with a stopcock and water manometer. Pressures above 25cm H2O are associated with decreased organ perfusion.
Image by Narupon Promvichai from Pixabay
Spontaneous hemoabdomen:
This category is distinct from other common causes of a hemoabdomen. Obtaining a thorough history and point-of-care diagnostics can quickly decrease the suspicion of a traumatic hemoabdomen or coagulopathy. Often with a traumatic hemoabdomen, the patient presents with a recent history of trauma, such as vehicular trauma. Physical examination findings can also increase the suspicion for an unwitnessed trauma, such as bruising, fractured ribs, or skin abrasions or lacerations. Point of care diagnostics such as a PT clotting test (prothrombin time), can also be very helpful. A PT test that is normal or slightly elevated in the presence of a hemoabdomen would decrease the suspicion of the primary cause being a coagulopathy, as clinical experience would require a PT test to be out of range (or close to out of range) to increase the suspicion of the primary cause being a coagulopathy to result in a hemoabdomen. A slight elevation often can be considered a consumptive coagulopathy.
Once trauma and coagulopathic causes have been ruled out, especially in an older, and often large breed dog (although there are no studies to say smaller breed dogs are any different), the term spontaneous (or non-traumatic, non-coagulopathic) hemoabdomen can be used.
There are several studies that have evaluated the spontaneous (non-traumatic, non-coagulopathic) hemoabdomen. These studies indicate an overwhelming likelihood neoplasia as an underlying cause, most commonly a ruptured splenic hemangiosarcoma (65-85%). Other causes do exist, both benign (ruptured hematoma) and malignant (e.g. mesothelioma, carcinoma, pheochromocytoma, lymphoma), but unfortunately the overwhelming likelihood is that a spontaneous hemoabdomen in an older dog will result from a splenic hemangiosarcoma.
Often these patients present in shock, specifically hypovolemic shock. Physical examination findings may include tachycardia, poor pulses, pale mucous membranes, increased respiratory rate and effort, and a distended abdomen with a palpable fluid wave. As in other causes of hemoabdomen, the first priority should be stabilization (e.g. intravenous catheter placement, fluid therapy, oxygen therapy, etc). Based on the patient’s state of illness, fluid therapy options to debate would include isotonic crystalloids, hypertonic saline, colloids, and even blood products.
Diagnostic work up
Following diagnosis and stabilization, as these are often older dogs with a primary concern for a neoplastic process, diagnostics that to considered should include:
- Bloodwork (CBC and Chemistry Screen) – to check for cell counts, organ values, electrolytes, and overall assess for metabolic or electrolyte derangements which would need correction
- Coagulation testing (specifically a prothrombin time – PT) – this should have been performed in the initial diagnostics on presentation to place the patient in this specific category (non-traumatic, non coagulopathic) – but if not, should be performed pre-operatively.
- Thoracic X-Rays – While helpful to assess cardiac size and shape, often the primary reason to recommend thoracic x-rays is to identify pulmonary metastasis. The presence of pulmonary metastasis would worsen the prognosis substantially and likely make this patient a poor candidate for surgery and anesthesia.
- Abdominal Ultrasound – My personal experience with an abdominal ultrasound and interpretation for clients falls in 1 of 3 scenarios:
-
- There is a solitary mass (spleen, liver, etc) that can be identified. Often radiologists are reluctant (and refuse) to note their impression of malignancy and while not helpful in differentiating between a benign or malignant tumor for the owners in their decision, a solitary mass present would hopefully lead one to assume this patient is a better surgical candidate in the absence of diffuse disease. The owner must also understand that there is a possibility that microscopic disease exists (not able to be seen on ultrasound) which may be identified during the exploratory procedure.
- There are multiple masses present (not just on one organ). While malignancy cannot be confirmed, the presence of multiple masses throughout the abdomen would give the impression that malignancy is more likely, and this patient is likely a worse surgical candidate than the previous patient with one solitary mass.
- No masses/lesions have been identified. At that time, further investigation is warranted (unwitnessed trauma?) and further stabilization may be needed to note progression.
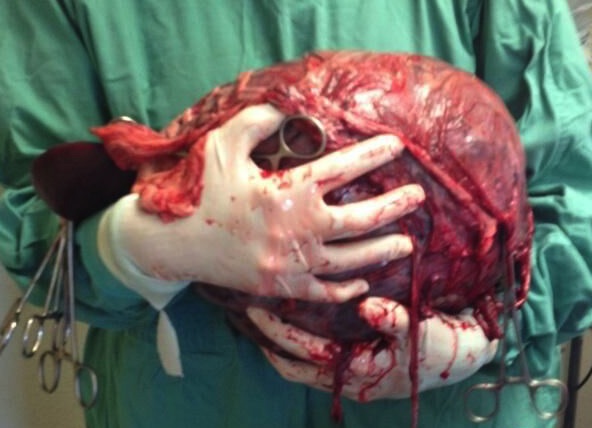
Does every patient need an ultrasound? I have clients that would like to save their pet regardless of the ultrasound findings. Are the ultrasound findings then academic in nature? If the client understands the risk that diffuse disease may be present and identified during surgery, resulting in a phone call to discuss humane euthanasia on the table, wouldn’t it then be reasonable to save the $400-$600 on the ultrasound and proceed directly to surgery following stabilization and additional diagnostics? Ultimately, once stabilized to the best of the clinician’s ability, an exploratory laparotomy is needed.
Learn more in Part 2 of “Emergency Room Approach to Anemia” in a few weeks HERE!
References (For Part 1 and 2):
- Aronsohn MG, Dubiel B, Roberts B, Powers BE.Prognosis for Acute Nontraumatic Hemoperitoneum in the Dog: A Retrospective Analysis of 60 Cases (2003–2006). Am. Anim. Hosp. Assoc. 2009 March/April; 45:72-77.
- Brockman DJ, Mongil CM, Aronson LR, Brown DC.A practical approach to hemoperitoneum in the dog and cat. Vet Clin North Am Small Anim Pract. 2000 May; 30(3):657-68. Review.
- Clifford CA, Pretorius S, Weisse C,et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med 2004;18:330–338
- Croce MA, Fabian TC,et al. Presley Regional Trauma Center, Department of Surgery, University of Tennessee-Memphis, USA. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995 Jun; 221(6):744-53; discussion 753-5.
- Delgado Millán MA, Deballon PO. Department of Surgery, Hospital Universitario de Getafe, Spain. Computed tomography, angiography, and endoscopic retrograde cholangiopancreatography in the nonoperative management of hepatic and splenic trauma. World J Surg. 2001 Nov; 25(11):1397-402.
- Feldman BF, Zinkl JG, Jain NC, eds.Schalm’s Veterinary Hematology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2000.
- Hammer AS, Couto CG, Swardson C, Getzy D. (1991),J Vet Intern Med, Vol.5, No. 1, pp. 11-14.
- Hammond TN, Pesillo-Crosby SA.Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003-2005). J Am Vet Med Assoc. 2008 Feb 15; 232(4):553-8. Erratum in: J Am Vet Med.
- Hargis AM, Feldman BF. (1991),JAVMA, Vol. 198, No. 5, March, pp. 891-894.
- Herold LV, Devey JJ, Kirby R, Rudloff E. Clinical management of hemoperitoneum in dogs.J Vet Emerg Crit Care 2008;18(1):40–53.
- Hopper K, Bateman. An updated view of hemostasis: mechanisms of hemostatic dysfunction associated with sepsis.J Vet Emerg Crit Care 2005; 15(2): 83-91
- Levinson JG, Bouma JL, Althouse GC, Rieser TM.Prevalence of malignancy when solitary versus multiple lesions are detected during abdominal ultrasonographic examination of dogs with spontaneous hemoperitoneum: 31 cases (2003-2008). J Vet Emerg Crit Care (San Antonio). 2009 Oct; 19(5):496-500.
- Millis DL, Hauptman JG, Fulton RB. (1993),Vet Surg, Vol. 22, No. 2, pp. 93-97.
- Mongil CM, Drobatz KJ, Hendricks JC. University of Pennsylvania, Philadelphia.Traumatic hemoperitoneum in 28 cases: a retrospective review. J Am Anim Hosp Assoc. 1995 May-Jun; 31(3):217-22.
- Nakamura K, Sasaki K, Murakami M,et al. Contrast-enhanced ultrasonography for characterization of focal splenic lesions in dogs. J Vet Intern Med 2010;24:1290–1297.
- Pintar J, Breitschwerdt EB, Hardie EM, Spaulding KA.Acute Nontraumatic Hemoabdomen in the Dog: A Retrospective Analysis of 39 Cases (1987–2001). Am. Anim. Hosp. Assoc. 2003 November/December; 39:518-522.
- Scott-Moncreiff JC, Treadwell NG, McCullough SM, Brooks MB. (2001),JAAHA, Vol. 37, pp. 220-227.
- Smith SA, The cell based model of coagulation.J Vet Emerg Crit Care 19(1) 2009: 9-10
- Sorenson SR, Rozanski EA, Tidwell AS,et al. Evaluation of a focused assessment with sonography for trauma protocol to detect free abdominal fluid in dogs involved in motor vehicle accidents. J Am Vet Med Assoc 2004;225:1198–1204.
- Streeter EM, Rozanski EA, de Laforcade-Buress A, Freeman LM, Rush JE. Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA.Evaluation of vehicular trauma in dogs: 239 cases (January-December 2001). J Am Vet Med Assoc. August 2009; 235(4):405-8.
- Thrall MA,et al. Veterinary Hematology and Clinical Chemistry. Philadelphia: Lippincott Williams & Wilkins, 2004.
Only VETgirl members can leave comments. Sign In or Join VETgirl now!