September 2023
By Dr. Garret Pachtinger, VMD, DACVECC, Director of Operations / Co-Founder, VETgirl
Cardiac Emergencies in the ER: Part 1 with Dr. Garret Pachtinger
In Part 1 of a two-part VETgirl online veterinary continuing education blog, Dr. Garret Pachtinger, DACVECC talks about CHF, pericardial effusion, arrhythmias, and so much more! Instead of becoming tachypneic, make sure to check back in a few weeks to read Part 2 HERE.
Cardiac diseases commonly seen in the small animal emergency room include congestive heart failure (mitral or tricuspid regurgitation, hypertrophic cardiomyopathy, dilated cardiomyopathy), myocardial failure (dilated cardiomyopathy, end-stage heart disease), pericardial effusion, arrhythmias, and aortic thromboembolism in cats secondary to HCM. As heart failure will be discussed in another lecture, this lecture will focus on other cardiac emergencies.
Regardless of the presenting complaint, an important concept to remember when approaching any emergency patient is a rapid primary survey, keeping in mind the ABCDs of evaluation and resuscitation. Briefly, “A” refers to Airway or Arterial Bleeding. “B“, Breathing is equally important assessing the character of the patient’s respirations. “C” refers to Circulation and the overall perfusion status of the patient. Finally, “D” refers to Disability notably the patients mental status.
Emergency Therapy
Emergency management of the patient presenting with respiratory distress includes systemic oxygen delivery and minimizing patient stress. While flow-by and oxygen mask oxygen delivery will allow concurrent patient assessment, there are times when other methods of oxygen delivery are needed.
| Supplementation technique | Required flow rate | Maximum inspired oxygen concentration achieved |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Once initial patient assessment is made, a more thorough physical examination is essential in the diagnosis and management of emergency cardiac patient.
Cardiac Tamponade / Pericardial Effusion
Cardiac tamponade results from the pressure of pericardial effusion on the heart leading to decreased filling, decreased cardiac output, and ultimately left and right heart failure. The degree of pressure exerted by the pericardial effusion depends on several factors. These include the volume of pericardial effusion, the rate of pericardial fluid accumulation, and the distensibility of the fibrous pericardium. In the author’s opinion, there are two common presentations of pericardial effusion. Patients presenting with acute cardiac tamponade often have a small volume of pericardial effusion (50–100 ml) which causes marked intrapericardial pressure and cardiac tamponade. However, we do also see patients with a more chronic, slower accumulation where there is increased compliance, allowing the pericardial sac to accommodate a significantly larger amount of fluid before intrapericardial pressure increases enough to result in cardiac tamponade.
Clinical signs of patients suffering from pericardial effusion may include tachycardia, tachypnea, poor or absent femoral pulses, pulsus paradoxus, jugular venous distension, dull heart sounds, exercise intolerance, weakness, and syncope. If more chronic in nature, patients may display signs of right-sided congestive heart failure including hepatomegaly, ascites, and jugular venous distension.
Aside from the traditional diagnostics (e.g. routine bloodwork and thoracic radiographs), echocardiography is recommended for the diagnosis of pericardial effusion. Pericardial effusion is diagnosed by the presence of hypoechoic fluid between the epicardium and the pericardium.
Diagnostic and therapeutic pericardiocentesis is indicated in patients with pericardial effusion. Equipment needed for pericardiocentesis include clippers, antimicrobial scrub, 70% ethyl alcohol, sterile drapes, sterile gloves, electrocardiogram (ECG), ultrasound (if available), large intravenous catheter or pericardiocentesis catheter, extension set, three-way stopcock, syringe, sampling tubes (red top and EDTA) and 2% lidocaine (both for local analgesia and preparedness if ventricular tachycardia develops.)
To perform a pericardiocentesis, the patient is placed in sternal recumbency or lateral recumbency. Anesthesia is not necessary although sedation with an opioid/diazepam combination can be helpful for mild chemical restraint. A local block with 2% lidocaine can be used to reduce discomfort as well. Cardiovascularly compromising medications such as propofol, acepromazine, and inhalant anesthesia should be avoided. Unless ultrasound guidance dictates a more appropriate location, the patient is prepared by clipping and scrubbing between the 4th and 6th intercostal space. While there is controversy as to the best side to use, the author prefers to enter the right side of the thorax. Similar to the thoracocentesis discussed above, the needle should enter cranial to the rib as the intercostal vessels and nerve runs caudal to the rib.
At the preference of the clinician, to prevent drag of the catheter through the skin a small skin stab incision can be made with a No. 11 scalpel blade. Also, at the preference of the clinician, side holes can be placed in the distal portion of the pericardiocentesis catheter. If side holes are made, avoid a hole greater than 40% of the circumference of the catheter and holes directly opposite each other on the catheter, both which increase the risk of catheter weakness.
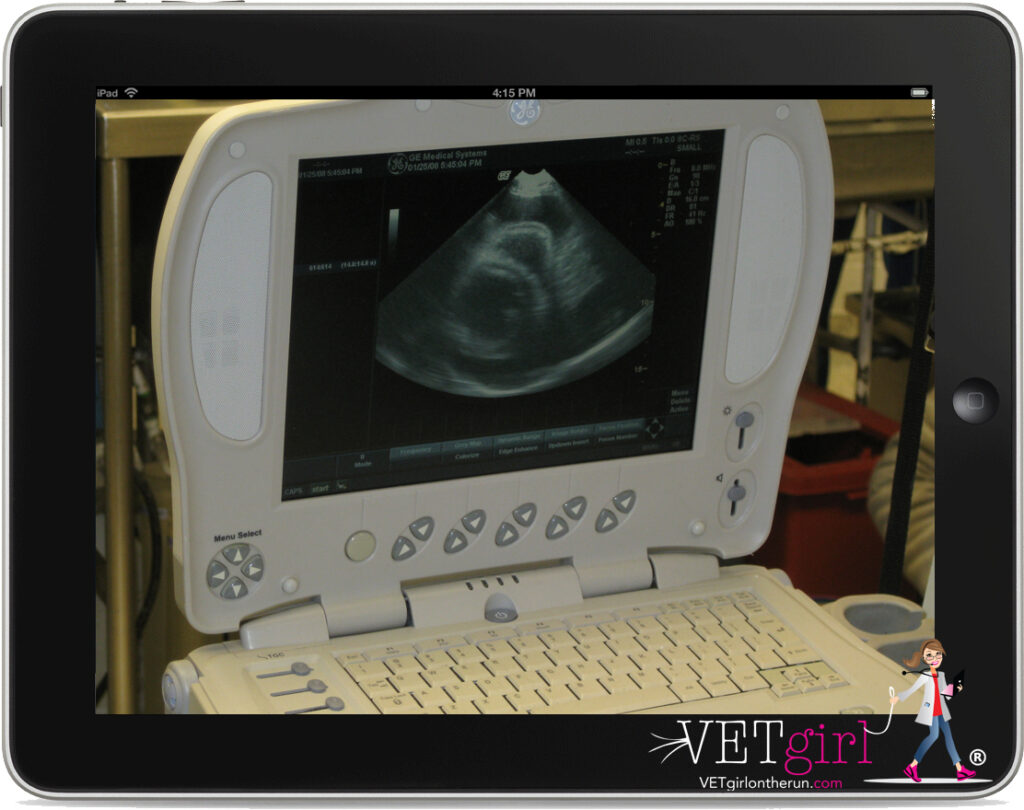
With appropriate patient monitoring including ECG, the catheter is inserted through the skin and into the pleural space. Once within the pleural space, the catheter is advanced slowly (1-2mm at a time) towards the heart while continuously monitoring the patient for discomfort and the ECG for arrhythmias. As the catheter is advanced, the clinician is watching carefully for fluid accumulation into the hub of the catheter. Typical fluid from the pericardial space will range from red to a port wine color. Once the fluid is seen within the hub of the catheter, the catheter is advanced another 1-2mm to make certain it is best seated within the pericardial space. The stylet is then removed, and the catheter is connected to the extension tubing along with a three-way stopcock. Using a 10-20ml syringe, the fluid is aspirated. A sample of the aspirated fluid is placed into a red top tube and a lavender top tube for further analysis. Specifically, the red top tube is monitored for clotting. A clot within the red top tube is a concern for trauma to the heart via the catheter and the catheter should be removed from the pericardial space. The amount of fluid obtained will vary but may be as much as 1/2 to 1 liter in a large breed dog. As they are often tachycardic on presentation, the clinician should notice a fairly dramatic decrease in heart rate within a few minutes of successful pericardiocentesis.
Life-Threatening Arrhythmias
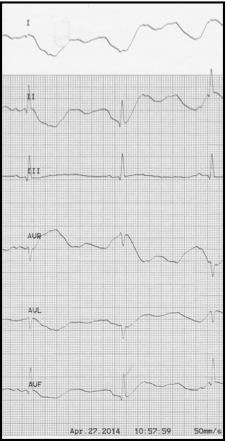
The most common arrhythmia the small animal veterinarian will see is a tachyarrhythmia. These are also considered to be the most concerning as tachyarrhythmia’s require increased oxygen consumption and lead to reduced diastolic filling and coronary artery perfusion. Underlying causes of tachyarrhythmia’s include shock, anemia, hypoxia, hyperthyroidism, infection, inflammation, and pain. Supraventricular tachycardias should improve with treatment and resolution of the underlying cause (i.e. fluid therapy for hypovolemic shock or oxygen therapy for hypoxemia). If the heart rate does not decrease with appropriate therapy, a vagal maneuver can be attempted by applying pressure to the eyes or carotid sinus pressure. If there is no improvement despite appropriate therapy and despite a vagal maneuver, drug therapy is considered, notably digoxin. Other antiarrhythmics which may be effective include propranolol (20-60 mcg/kg IV slowly over 5-10 min.) or verapamil (.05 mg/kg IV q 10-30 min, up to 3 times). Both of these are negative inotropes and should be used with caution if there is concurrent evidence of congestive heart failure. Intravenous diltiazem (0.25 mg/kg administered slowly over 3 minutes) can be used instead of verapamil to control supraventricular tachycardias.
Ventricular tachycardia is another common arrhythmia seen, associated with primary cardiac disease or secondary to systemic disease. The arrhythmia is treated pharmacologically if signs of hemodynamic instability are present, notably with EKG findings including tachycardia (>160bmp), multiform QRS configurations, R on T phenomenon, and or hypotension. Lidocaine is the drug of choice for ventricular arrhythmias, dosed initially with a bolus of 2-4mg/kg IV given slowly to effect while monitoring the electrocardiogram. This bolus is followed by a CRI (25-80 μg/kg/min). Refractory ventricular arrhythmias can be treated with procainamide (2-15 mg/kg IV over 20-30 minutes).
Bradyarrhythmias are not as commonly seen in clinical practice, although bradycardia as a result of hyperkalemia seen with (feline) urethral obstruction is often seen. Aside from hyperkalemia as a result of urethral obstruction in male cats, other common causes include hypoadrenocorticism and renal failure. Treatment will depend on the underlying cause, but for hyperkalemia may include fluid therapy, Calcium gluconate (0.2-0.5 ml/kg IV), regular insulin (0.25 U/kg IV), dextrose (0.5g/kg), or sodium bicarbonate (1-2 mEq/kg IV slowly).
Aortic / Arterial Thromboembolism
Arterial thromboembolism (ATE) is a condition associated with high morbidity and mortality in cats, most commonly with an acute presentation resulting from an underlying cardiomyopathy. Common historical findings and examination findings include loss of peripheral pulses, tissue pallor, lower motor neuron signs and cool extremities in the presence of pain. These patients may also be hypothermic, with a lower rectal temperature. Of cats treated for ATE in general practice, survival to 7 days is estimated at 55%. Of those cats alive at 1 week, 1-year survival was 20%. (Borgeat et al. 2012).
Treatment for ATE patients may include:
- Oxygen therapy and diuretic therapy (e.g. furosemide) as the common theme is an underlying cardiomyopathy and patients with ATE may present in CHF as well.
- Analgesia (e.g. buprenorphine, torbutrol, fentanyl, etc.)
- Aspirin or clopidogrel.
Congestive Heart Failure:
Emergency Therapy
Emergency management of the patient presenting with congestive heart failure and respiratory distress includes systemic oxygen delivery and minimizing patient stress. While flow-by and oxygen mask oxygen delivery will allow concurrent patient assessment, there are times when other methods of oxygen delivery are needed. Once initial patient assessment is made, a more thorough physical examination is essential in the diagnosis and management of emergency cardiac patient.
Myxomatous mitral valve disease (MMVD) is the most common cause of congestive heart failure in dogs. With that said, congestive heart failure (CHF) does not simply imply there is a cardiac abnormality (such as MMVD), rather CHF must be considered a clinical syndrome characterized by specific signs including tachypnoea/dyspnea as a result of pulmonary edema, pleural effusion, ascites) or other examination and diagnostic abnormalities.
Clinical Signs
Patients with congestive heart failure often present in respiratory distress. Common examination findings include an increased respiratory rate and effort. If pulmonary edema is present, auscultation commonly is reported to have pulmonary crackles. More common in feline patients, dull lung sounds may be present ventrally with pleural effusion. Ascites may also be present with right sided heart failure as a result of tricuspid regurgitation, DCM, or heartworm disease. Other common physical examination findings include auscultation of a heart murmur, hypothermia in cats, pale mucous membranes, and other signs of respiratory distress (i.e. extension of the head/neck abduction of the elbows, and reluctance to lay down). Although uncommon in dogs, absent femoral pulses, cold rear extremities, and hind limb paresis are seen with aortic thromboembolism (ATE), seen most commonly as a consequence of hypertrophic cardiomyopathy (HCM) in cats.
Diagnostic Approach
Along with the history and physical examination, diagnostics to consider include blood pressure, pulse oximetry, thoracic radiographs, or thoracic ultrasound. Before performing diagnostics, it is important to make sure the patient is stable and can tolerate the diagnostics without a risk of decompensation.
Thoracic radiographs are often considered the mainstay diagnostics in evaluating the heart and lungs. In fulminant congestive heart failure, radiographs commonly show congestion / distension of the pulmonary vessels and interstitial to alveolar pulmonary infiltrates. In dogs, the pulmonary interstitial to alveolar disease is often seen in the perihilar area while cats may have a more generalized pulmonary patent of edema. Specific cardiac disease may also become more apparent with the use of radiographs, notably a large, globoid heart with dilated cardiomyopathy (DCM) or pericardial effusion. When performing thoracic radiographs, at least two views should always be taken with many cardiologists preferring a lateral view and dorsoventral (DV) view.
While thoracic radiographs often confirm the diagnosis of CHF, thoracic ultrasound is an upcoming diagnostic in the ER. Along with the TFAST and AFAST , a new term, “Vet Blue” has recently been discussed. Using these ultrasound techniques, lung pathology is assessed based on the distinction between wet (ultrasound lung rockets (ULRs) vs. dry lung (A-lines with a glide sign). The goal of using this technique is to provide rapid, point-of-care global evaluation of the emergent patient with minimal restraint and risk of decompensation. For the TFAST (Vet Blue) procedure, the patient is placed in either right lateral recumbency and/or sternal recumbency. Dorsal recumbency is not recommended as it has not been validated for VetBlue and it also may increase patient stress. The Vet Blue “L”ung Scan (VBLS, and “blue” for cyanosis, “L” for the scan pattern) is a rapid respiratory evaluation primarily based on the concept of wet vs. dry lung. In human patients lung ultrasound has been shown to be superior to chest auscultation for the detection pulmonary pathology. The goal of the VBLS scan is to identify pulmonary patterns that improve the speed of diagnosis for the respiratory distressed patient preempting the stress of thoracic radiography.
Acute Management
The initial treatment of congestive heart failure will vary slightly depending on the patient as well as specific diagnosis, but mainstay therapies typically involve oxygen and furosemide (1-4 mg/kg IV as often as every 1-2 hours initially for fulminant edema) as well as monitoring including blood pressure, pulse oximetry, hydration status, electrolyte status, and renal status.
Additional therapeutic options may include:
- Nitroglycerine ointment 1/8-1/4 inch q8-12h
- Oral hydralazine (1 to 2 mg/kg PO q8–12h)
- Sodium nitroprusside – starting at 0.5 micrograms/kg/minute CRI
- Pimobendan 0.2-0.3mg/kg POq12
Pimobendan is a phosphodiesterase-III inhibitor that sensitizes the myocardium to calcium and improves inotropic activity in addition to causing arteriolar and venous dilation. In addition to its use as a long-term inodilator in the treatment of dogs with CHF, Pimobendan is also recommended for use in emergency therapy of CHF, as it can have an onset of effects within one hour.
In cases of low output failure (weak pulses, pale membranes, slow CRT, weakness, hypothermia, azotemia), dobutamine is a synthetic beta-adrenergic agonist is considered. This is commonly used in patients with DCM. Dobutamine has a dose range of 2–20 mcg/kg/minute at lower doses, dobutamine improves cardiac contractility with minimal effects on chronotropy or heart rate. At higher doses, however, dobutamine can be pro-arrhythmogenic.
Summary
Patients presenting with evidence of emergent cardiac disease should be triaged quickly and treated immediately to reduce morbidity and mortality. Oxygen is a mainstay therapy for cardiac patients and should be administered on presentation and during the initial assessment phase. Prognosis will vary on the underlying cause of disease although patients may live for several years with careful monitoring.
But wait, there’s more. Make sure to check back in a few weeks to read Part 2 HERE. Because it’s really a heart-stopper!
| Key Drug | Drug Class | Dose Range | Frequency | Route | Indications |
| Furosemide | Diuretic | 2-4 mg/kg | Every 2-4 hours as needed, then every 8 hours | IV best in patients with CHF; IM | Pulmonary edema due to CHF |
| Nitroglycerin | Vasodilator | 1/8″ strip on ear pinnae | Every 6 hours | Topical | Vasodilation to decrease afterload on the heart |
| Dobutamine | β-1 agonist | 2-10 μg/kg/min | CRI | IV | Positive inotrope to increase cardiac output in patients with primary myocardial failure |
| Lidocaine | Class I antiarrhythmic | 40–80 mcg/kg/min | CRI | IV | Ventricular tachycardia |
| Procainamide | Class I antiarrhythmic | 25–40 mcg/kg/min | CRI | IV | Ventricular tachycardia |
| Diltiazem | Calcium channel blocker | 0.25 mg/kg slow bolus | Q 20 min | IV | SVT +/- atrial fibrillation |
| Esmolol | Beta-blocker | 0.01 mg/kg slow bolus | Q 5 min | IV | SVT |
| Nitroprusside | Nitrate | 2 to 10 mcg/kg/min | CRI | IV | Refractory CHF |
References
- Andreka P, Frenneaux MP. Haemodynamics of cardiac arrest and resuscitation.Curr Opin Crit Care. 2006;12(3):198–203.
- Berg RA, Otto CW, Kern KB,et al. A randomized, blinded trial of high-dose epinephrine versus standard-dose epinephrine in a swine model of pediatric asphyxial cardiac arrest. Crit Care Med. 1996;24(10):1695–1700.
- Berg RA, Otto CW, Kern KB,et al. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study.Crit Care Med. 1994;22(2):282–290.
- Buckley GJ, Rozanski EA, Rush JE. Randomized, blinded comparison of epinephrine and vasopressin for treatment of naturally occurring cardiopulmonary arrest in dogs.J Vet Intern Med. 2011;25(6):1334–1340.
- Fletcher DJ, Boller M, Brainard BM,et al. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 7: Clinical guidelines. J Vet Emerg Crit Care (San Antonio). 2012;22(Suppl 1):S102–131.
- Hopper K, Epstein SE, Fletcher DJ,et al. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 3: Basic life support. J Vet Emerg Crit Care (San Antonio). 2012;22(Suppl 1):S26–43.
- Iwami T, Kitamura T, Kawamura T,et al. Chest compression-only cardiopulmonary resuscitation for out-of-hospital cardiac arrest with public-access defibrillation: a nationwide cohort study. Circulation. 2012;126(24):2844–2851
- Kern KB, Hilwig R, Ewy GA. Retrograde coronary blood flow during cardiopulmonary resuscitation in swine: intracoronary Doppler evaluation.Am Heart J. 1994;128(3):490–499.
- Lindner K, Haak T, Keller A,et al. Release of endogenous vasopressors during and after cardiopulmonary resuscitation. Heart. 1996;75(2):145–150.
- Lindner KH, Dirks B, Strohmenger HU,et al. Randomised comparison of epinephrine and vasopressin in patients with out-of-hospital ventricular fibrillation. Lancet. 1997;349(9051):535–537.
- Mentzelopoulos SD, Zakynthinos SG, Siempos I,et al. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83(1):32–39.
- Paradis NA, Martin GB, Rivers EP,et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. J Am Med Assoc. 1990;263(8):1106–1113
- Rozanski EA, Rush JE, Buckley GJ,et al. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 4: Advanced life support. J Vet Emerg Crit Care (San Antonio). 28.
- Stiell IG, Hébert PC, Wells GA,et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105–109.
- Wik L, Naess PA, Ilebekk A,et al. Effects of various degrees of compression and active decompression on haemodynamics, end-tidal CO2, and ventilation during cardiopulmonary resuscitation of pigs. Resuscitation. 1996;31(1):45–57
- Borgeat K, Wright J, Garrod O, et al. Arterial thromboembolism in 250 cats in general practice: 2004–2012. J Vet Intern Med. 2012;28:102–108.
- Smith, F.W.K., Keene, B.W.: Left Sided Congestive Heart Failure. In The Five Minute Veterinary Consult, 3rd(eds. L. Tilley and F.W.K. Smith) Baltimore, Lippincott, Williams & Wilkins, 2004.
- Tilley, L.P., and Goodwin, J.: Manual of Canine & Feline Cardiology, 3rdPhiladelphia, W.B. Saunders, 2000.
- Norsworthy, G., Crystal, M., Fooshee, S., Tilley, L.P.: The Feline Patient.Baltimore, Lippincott, Williams & Wilkins, 1998.
Only VETgirl members can leave comments. Sign In or Join VETgirl now!
Great reference for oxygen rates and modes of therapy for cardiac patients as well as the information about what oxygenation level is achieved.
Love the references
Great references